Acid Base Reaction Net Ionic Equation
Acid Base Reaction Net Ionic Equation. In other words, the net ionic equation applies to reactions that are strong electrolytes in. Definition of an acid/base reaction;
The reaction of sodium hydroxide and acetic acid (also called ethanoic acid) represents a net ionic equation involving a strong base and a weak acid. Then n h x 4 o h, better described as a hydrate n h x 3 ⋅ h x 2 o, is added. H₃o⁺(aq) + oh⁻(aq) → 2h₂o(l).
Hno 3 + Naoh = Nano 3 + H 2 O Is A Neutralization Reaction.
The reaction of sodium hydroxide and acetic acid (also called ethanoic acid) represents a net ionic equation involving a strong base and a weak acid. Strong acid/strong base 1) hydrochloric acid and sodium hydroxide. Weak acids only dissociate partially and are not.
Ok, Notice How We Separate Each Substance With The (Aq) Designation Into Ions In Our Total Ionic Equation Above.
Finally, let’s write the net ionic equation. Strong acids and strong bases are considered strong electrolytes and will dissociate completely. A neutralization reaction takes place when the hydrogen ions in an acidic solution react with the hydroxide ions from a basic solution to form water.
But That's Not The Net Ionic Equation.
Constant and therefore the extent of reaction depends on the relative strength of the acids and bases in the reaction. A chemical reaction between an acid and a base is called as neutralization reaction. One of the most important reactions of acids and bases is their ability to neutralize one another.
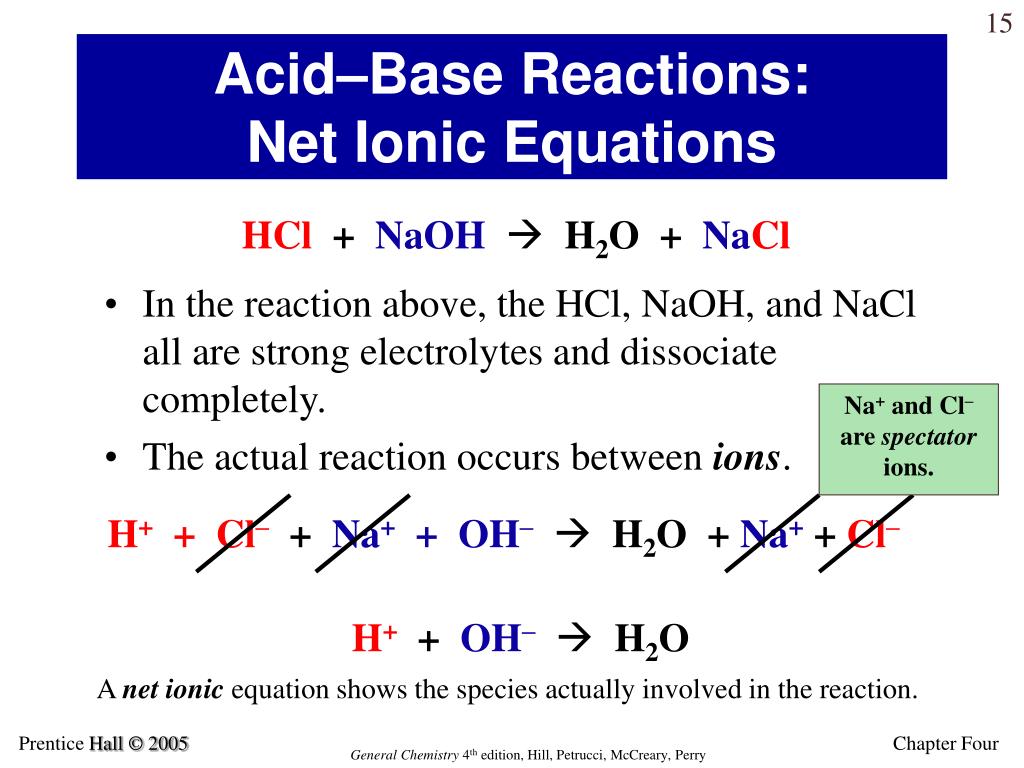
Remember, Spectator Ions Are Ions That Appear On Both Sides Of The Arrow Unchanged.
Write the net ionic equation that describes the reaction of a weak acid, acetic acid when reacts with an aqueous solution of the strong base strontium hydroxide to form ionic compound strontium and liquid water. For the ionic equation, you dissociate the strong acid or the strong base, and leave a weak acid or weak. The overall and net ionic equations for the four reactions are shown below in table 1.
The Rate Constant For The Rate Constant For.
In this case, an acid and a base produce a salt and water. This means that we will split them apart in the net ionic equation. To write the net ionic equation, we’ll cancel out all spectator ions.
Comments
Post a Comment